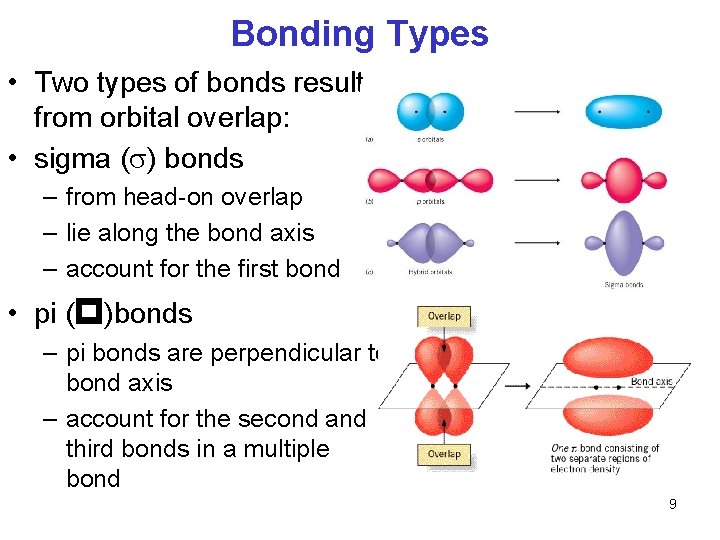
Describes Double and Triple Bonds Using Valence Bond Theory
Often the bonding atomic orbitals have a character of several possible types of orbitals. They result from side-to-side overlap of p-orbitals.
Chemical Bonding Valence Bond Theory In A Nutshell
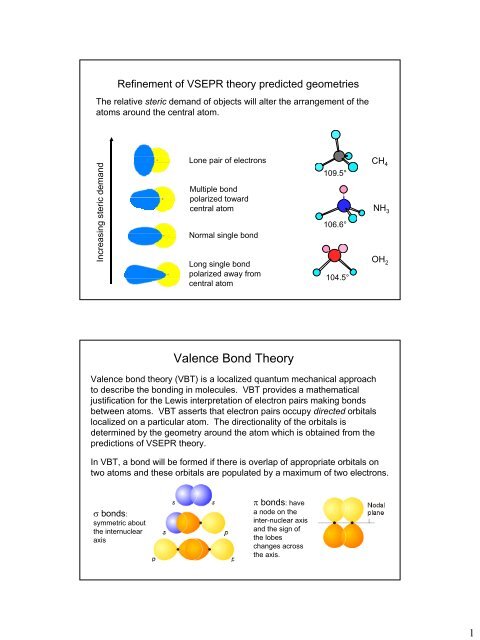
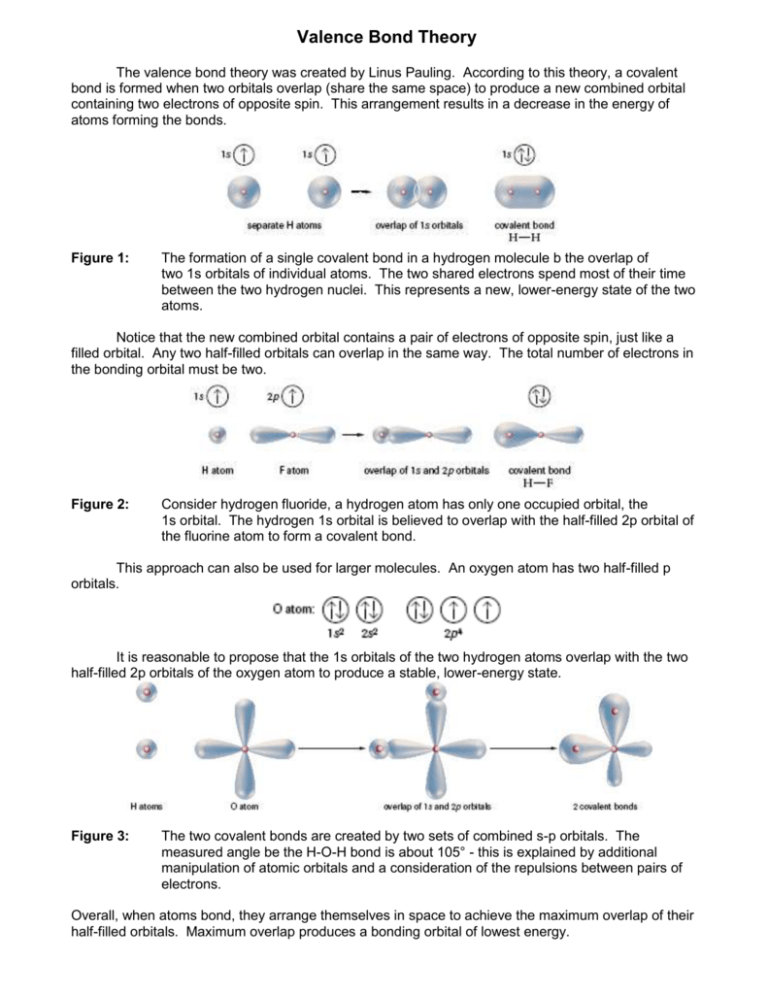
In chemistry valence bond VB theory is one of two basic theoriesalong with molecular orbital MO theorythat use quantum mechanics to explain chemical bonding.

. The theory helps explain bond formation in cases where a Lewis structure cant describe real behavior. In terms of bond order single bonds have one sigma bond double bonds consist of one sigma bond and one pi bond and triple bonds contain one sigma bond and two pi bonds. We say that orbitals on two different atoms overlap when a portion of one orbital and a portion of a second orbital occupy the same region of space.
In valence bond theory the pi bond between the two oxygen atoms in O2 results from. Which of the following correctly describes double and triple bonds using valence bond theory the localized electron modely They increase the distance between the bonded atoms. Valence Bond Theory 2.
They result from rotation of atoms around the bond axis. They are formed by interactions of s- and p-orbitals. The section below provides a more detailed description of these topics worked examples practice problems and a.
They help rotation of atoms around the bond axis. Compounds containing triple bonds σ 2π or adjacent double bonds CO 2 have sp hybridization. O Triangular bipyramidal Triangular pyramidal Triangular planar Tetrahedral Which of the following correctly describes double and triple bonds using valence bond theory the localized electron model.
Two in 2s and four in 2p. They have the same bond length as single bonds. The VB theory describes the formation of covalent.
When determining the coordinate number double bonds and triple bonds count as one bond. Bond Order of valence e in freeunbonded atom -1. 425 3605 Views.
Use valence bond theory to explain why rotation is possible around single bonds but not double bonds singlesigma bonds are formed from the end to end overlap of the orbitals. They weaken the bond. Valence bond theory explains howwhy covalent bonds form.
This section explores valence bond theory and how it is applied to molecules with multiple bonds and those that need resonance structures to better describe their bonding. They increase the distance between the bonded atoms. According to VB theory a covalent bond forms from the physical overlap of half-filled valence orbitals in two atoms.
Mechanism of Bonding in VB Theory. They have the same bond length as single bonds. Valence bond theory explains howwhy covalent bonds form.
When chemical bonds form between atoms the atomic orbitals may be hybrids of sigma and pi bonds. According to molecular orbital theory what must be true for an interaction to be strong between two overlapping. Thus the coordinate number of C is 2 and the corresponding hybridization is sp.
If waves interact destructively the resulting orbital is higher in energy. An antibonding molecular orbital. HCN thus has one single and one triple bond.
They weaken the bond. 45 Votes Describe HCN molecular bond by using Valence Bond Theory. Which of the following correctly describes double and triple bonds using valence bond theory the localized electron model.
1Which of the following correctly describes double and triple bonds using valence bond theory the localized electron. Problem Set 17 Valence Bond VB Theory. It holds that bonds form as the partially filled valence electron orbitals of nearby atoms overlap.
Between any two atoms the first bond formed will always be a σ bond but there can only be one σ bond in any one location. When rotated the overlap stays the same so the bond is not broken. Valence bond theory describes a covalent bond as the overlap of half-filled atomic orbitals each containing a single electron that yield a pair of electrons shared between the two bonded atoms.
Which of the following correctly describes double and triple bonds using valence bond theory the localized electron model. Valence Bond theory describes covalent bond formation as well as the electronic structure of molecules. It holds that bonds form as the partially-filled valence electron orbitals of nearby atoms overlap.
However the atomic orbitals for bonding may be hybrids. Triple bonds contain a sigma bond and two pi bonds. They result from side-to-side overlap of p-orbitals.
Double bonds contain a sigma bond and a pi bond. In valence bond theory double and triple bonds are formed. When they are rotated the overlap is broken and the bond is.
They result from side-to-side overlap of p-orbitals. Doublepi bonds are formed from the side to side overlap of the overlap. If waves interact constructively the resulting orbital is lower in energy.
O has six valence electrons. They result from rotation of atoms around the bond axis. In MO theory we invoke the wave nature of electrons.
In 5 sentences or less describe what valence bond VB theory is. O They weaken the bond. Because px orbital of C and N will form sigma bond this leaves with two N atom p-orbitals which form two mutually perpendicular pi bonds to the two atomic p orbitals on the C atom.
Notice that there is a double bond. In 5 sentences or less describe what valence bond VB theory is. The double bond consists of one σ bond and one π bond and the triple bond consists of one σ bond and two π bonds.
What is the term for a molecular orbital that is at a. The theory assumes that electrons occupy atomic orbitals of individual atoms within a molecule and that the electrons of one atom are attracted to the nucleus of another atom. They weaken the bond.
O They result from side-to-side overlap of p-orbitals. They impede rotation around the bond axis. They require the use of hybrid orbitals for all bonds.
They help rotation of stoms around the bond axis. Which of the following correctly describes double and triple bonds using valence bond theory the localized electron model. Expanded Valence For elements beyond the second period we found several examples where the central atom in a Lewis structure had greater than 8 electrons in the valence.
They result from side-to-side overlap of p-orbitals. They increase the distance between the bonded atoms. Problem Set 16 Valence Bond VB Theory Chem 105 1.
Now hybridize to explain the bonds. A bonding molecular orbital. Which of the following correctly describes double and triple bonds using valence bond theory the localized electron model.
We will first define what sigma σ and pi π bonds are.

Hybrid Orbitals Explained Valence Bond Theory Crash Chemistry Academy Chemistry Chemistry Help Science Chemistry

Comments
Post a Comment